Have Cheese-Chasing Mice Unlocked a New Oral Cancer Remedy?
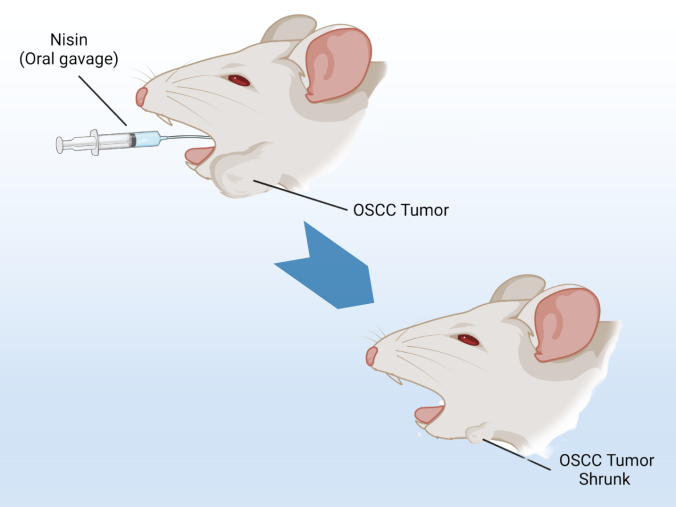
A five-year clinical trial led by UCLA School of Dentistry researchers and colleagues at UC San Francisco aims to test whether nisin, an antimicrobial molecule found in cheese, can be used to treat oral squamous cell carcinoma (OSCC).
Pre-clinical research helmed by the School’s Associate Dean of Research Dr. Yvonne Hernandez-Kapila has shown that nisin reduced the size of oral cancer tumors in mice and prolonged their survival, with no side effects or organ changes. Nisin preferentially kills oral cancer cells compared to normal cells, as well as bacteria causing periodontal disease, which is linked to oral cancer.
Funded through a five-year National Institute of Health/National Cancer Institute grant, Phase I/II trials will begin this month at the UCSF School of Dentistry, with data analysis conducted at UCLA. The study will determine if nisin can mitigate OSCC tumors (and their recurrence) and improve periodontal disease status in humans. OSCC has a poor five-year survival rate that has not improved in decades, and current treatments show limited effectiveness in late-stage disease. If successful, this therapy would mark a paradigm shift in cancer therapy focused on the use of antimicrobials and potentially reducing toxic side effects associated with traditional treatments.
“Who would have thought that a molecule from a probiotic bacteria found in cheese would help the fight against oral cancer,” Dr. Hernandez-Kapila, the Felix and Mildred Yip Endowed Chair, asked rhetorically. “To our knowledge, our lab was the first group to report this property in scientific literature and the first group implementing a clinical trial on this subject.”
In addition to potentially identifying nisin as a new treatment for oral cancer, researchers hope this new treatment modality will pave the way for the use of antimicrobials as novel therapeutic approaches for multiple forms of cancer. Since nisin has been tested in animals at high doses without any toxic effects, nisin treatment may address the critical need to mitigate the major side effects associated with traditional cancer treatments, like chemotherapy and radiation therapy.
With the successful completion of Phase I/II trials, a Phase III trial will compare the safety and effectiveness of nisin against the current standard of treatment. This phase would involve several hundreds to thousands of OSCC/oral cancer patients exhibiting different stages of the disease.
The study, funded by NIH R01 CA269950-03, has included several nisin-related patents filed with support from the UCLA Technology Development Group. The full project detail report can be viewed at reporter.nih.gov.
